Amazon has taken one more conceptual step toward an integrated system that can size up fashion customers and sell them tailor-made clothing.
Lithium Australia’s SiLeach on track for patents in 148 countries
SiLeach process after being given the thumbs up by the Australian Patent Office World Intellectual Property Organisation.
This triggered a rigorous examination by the Australian Patent Office, which almost a year later has reported that SiLeach is indeed “novel, inventive, industry applicable and patentable”. Continue reading “Lithium Australia’s SiLeach on track for patents in 148 countries”
Genentech Defends Patents on Bevacizumab, Rituximab in US, Japanese Courts
Innovator drug developer Genentech, maker of the biologic therapies Avastin (bevacizumab) and Rituxan (rituximab), faces challenges from biosimilar developers in the United States and abroad. This week saw developments in cases concerning both molecules in US and Japanese courts of law. Continue reading “Genentech Defends Patents on Bevacizumab, Rituximab in US, Japanese Courts”
Apple Fails to Make the Top 10 on List of Companies With the Most Patents
What’s the most innovative company in America? You probably wouldn’t guess IBM, but it received more patents from the U.S. patent office than any other company in 2017, and it has topped the list for the past 25 years, according to an annual ranking just released by IFI Claims Patent Services. Patents have come under fire in recent years as companies sought to buy them up and then use them to bludgeon one another in court. Nevertheless, patents remain the single best measure we have of a company’s commitment to researching and developing new ideas, and bringing those ideas to market. Continue reading “Apple Fails to Make the Top 10 on List of Companies With the Most Patents”
Brazilian PTO Considers Automatically Granting 231,000 Patents to Get Rid of Backlog
The Brazilian Government is considering the adoption of an emergency measure to eliminate the Patent Office chronic backlog problem by automatically granting, without examination, 231,000 pending applications until 2020. The emergency measure has been labelled by the Government as an “extraordinary solution” and a draft of the plan was introduced for public discussion. Companies may soon need to deploy a strategy within a time-frame as short as 90 days to take full advantage of the new system while minimizing potential risks.
Brazilian PTO backlog
For the past 15 years, Brazil has been enduring one of the world’s most severe patent backlogs. The problem has grown considerably after the enactment of the 1996 Patent Statute, which was adopted to make the country TRIPS compliant. The amount of patent filings skyrocketed ever since, as patents were now available to fields not previously patentable, such as pharmaceutical technologies. Hence, in a 15 years period, the amount of applications rose up to about 200%, without a correspondent increase in the number of examiners within the Brazilian PTO.
As a result, the current waiting time averages in Brazil surpass the 10 years mark for nearly all technological fields. The overall average consists of 10,23 years from filing until grant, but some areas bear a much graver backlog. As a matter of fact, the backlog for applications in the telecom field reaches an astonishing 13,74 years average. At its turn, pharmaceutical applications do not fall short, as the average waiting time reaches 13,27 years for traditional drugs and 12,04 years for biopharmaceuticals.
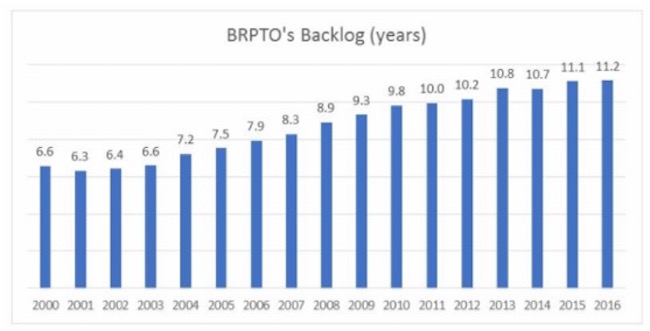
Although apparently discouraging, those figures actually show an improvement, as the overall average has once surpassed the 11 years mark. Fortunately, the current administration of the patent office and the federal government are committed to solving the problem and many measures were adopted to minimize its effects, such as hiring new examiners and the creation of fast track examination lines. Recently, though, the Brazilian PTO has introduced for discussion an unprecedented proposal to solve the problem for good.
The PTO and the federal government have proposed an abrupt measure which consists of automatically granting 231,000 pending non-pharmaceutical patent applications as currently drafted, with no examination whatsoever.
According to the PTO’s calculations, the current number of examiners would be sufficient to deal with the present amount of new filings, if it was not for the historical backlog the examiners must face. The Brazilian PTO currently relies on 326 examiners, whose productivity per capita has increased in recent years. Without the special measure under discussion, authorities estimate that the Brazilian PTO would take about thirteen years to get rid of the backlog, without considering new filings in that period.
Hence, the proposal is to eliminate the past backlog altogether, by granting the pending non-pharmaceutical applications in an automatic fashion, thus leaving the examiners solely dealing with new applications. In other words, the PTO has admitted the absence of any other viable option to reduce the backlog in the short run, since hiring a significant amount of new examiners was put out of the options because of the high costs involved and because it would not be legally possible to fire the exceeding examiners after the problem was solved.
So far, the Brazilian PTO has not provided many details and timing for the measure, but the basic lines of the plan were made available for public discussion. The Office is receiving contributions from the public, but it has made clear that suggestions that add complexity to the proposed procedure and involve human intervention are unlikely to be accepted, e.g., allowing claim amendments before the automatic grant or the request for substantive examination after grant apart from the standard post grant opposition procedure already in place.
We outline the basic rules of the proposed measure alongside our comments below.
Automatic grant streamline
In short, the proposal consists of a simplified examination procedure, according to which the Patent Office would automatically grant applications filed until a date yet to be established, against which no pre-grant oppositions had been filed and that have not been examined yet.
The measure would not include pharmaceutical applications, and neither divisional applications whose parent application had been already examined. Applications with the payment of annuities behind schedule would also be excluded from the program.
Applicants would be entitled to opt-out from the special measure upon filing a petition requesting so for each specific application, and third parties would have a 90-days term to file pre-grant oppositions after an application enters the line for automatic grant, which would also guarantee that the opposed applications would be examined.
The exclusion of pharmaceutical applications has a straight connection with the Brazilian procedure for granting patents in this field, as the national Food and Drug Agency – ANVISA takes part in the process by yielding its prior consent to those applications. Therefore, the PTO could not automatically grant pharmaceutical patents without securing ANVISA’s consent, which would be likely denied as the agency usually is concerned with the government’s policy towards public spending with the purchase of drugs for the country’s universal health care system.
The lack of definition regarding the coverage of the program, which would include applications filed until a given date, should motivate applicants to consider anticipating the entrance into the national phase of PCT applications in Brazil, as well as national filings claiming Paris Convention priority. Hence, it may well worth entering the national phase before PCT’s 30-months deadline or filing a new application before the Paris Convention 12-months deadline for claiming priority, since the application could fall within the automatic grant line.
Furthermore, bearing in mind that the Brazilian PTO may disregard any post-filed amendments, it is important to make sure that the desired coverage was provided by the original claims. Finally, applicants must do their best to secure the payment of annuities in due term, in order to avoid an involuntary exclusion from the program.
Strategic use of the opt-out option
Naturally, the automatic grant proposal is already spurring controversy. On one hand, it is held that the backlog would come to an end, but on the other hand, most patent practitioners envisage that patents subject to the special measure would be more vulnerable to challenges both before the Brazilian PTO, in post-grant opposition, and the Federal Courts, in invalidity lawsuits.
Moreover, patents granted under the program may deal with suspicion from state courts in enforcement procedures, as the judges may feel reluctant to grant injunctive relief for patentees whose patents were not properly examined by the PTO.
Hence, a strategic approach to the automatic grant procedure may include, for example, the use of the opt-out system mentioned above for particularly relevant applications, in order to let them follow the regular flow of substantive examination and, thereby, reduce the vulnerability of the patent once granted. Since the program is expected to significantly decrease the backlog, as most pending applications will be automatically granted, one might suppose that applications using the opt-out system will experience a faster examination as part of the global reduction of the backlog.
Watch over competitors
Last but not least, since the program allows companies to withdraw third parties’ applications from the automatic grant line by simply filing pre-grant oppositions against them, it is absolutely indispensable to check the portfolio of competitors for relevant applications in order to prevent them from being granted without examination.
Data extracted from our proprietary data-mining software reveal that pre-grant oppositions are usually able to reduce the chances of an application being granted by nearly 40%. Therefore, opposing an application could not only prevent the automatic grant thereof, but also effectively contribute to prevent its grant whatsoever.
Conclusion
Backlog has been cursing the Brazilian patent system for more than a decade. Its huge numbers could probably frighten away applicants, but there are effective ways to overcome it.
While the backlog is undeniably a complicated and grave problem, it equally affects all competitors in a particular industry and dealing with it in a strategic way is possible, either by taking advantage of an extended protection term or making use of the fast track examination procedures available at the PTO, which can certainly make a difference amongst competitors.
The Brazilian Patent Statute establishes an automatic extension in the term of validity of a patent in cases where the Patent Office takes more than a decade to grant the application. Accordingly, the protection is granted for 20 years counting from the filing date or 10 years counting from the date of grant, whichever the longest. This provision, however, is currently under attack in the Brazilian Supreme Court, where generic drugs companies claim its unconstitutionality.
Regardless of the term extension referred to above, the PTO offers some administrative paths to put an application into an examination fast track, which could be very effective. Amongst others, there are Patent Prosecution Highway (PPH) partnerships in force with the USPTO, EPO and JPO; a special program aimed at environmentally friendly technology applications called “Green Patents” program; and an expedited examination line for applications being infringed by third parties. Finally, even lawsuits against the patent office grounded on the unlawfulness of the backlog are also available and have provided favorable results over the past years in compelling the PTO to decide over applications by means of a court order.
Notwithstanding the foregoing, as a last resort to cope with the huge backlog of the past years, the Brazilian PTO and the federal government have proposed a special procedure to automatically grant non-pharmaceutical patent applications, as outlined above.
As seen in this paper, if the automatic grant is eventually approved and comes into force, strategic planning is essential to take advantage of the program while minimizing potential risks. Since the time-frame to deploy such strategy could be as short as 90 days, applicants should carefully review their own Brazilian portfolio, as well as their competitor’s, in order to develop the best approach in advance.
Source IP Watchdog
Cannabis at the Cutting Edge – Patents for a New U.S. Industry
When most people think of patented technologies, they think of cell phones, computers, and pharmaceuticals. What entrepreneurs in the cannabis industry may not know is that cannabis and cannabis-related products are also patentable. Patents are important for any business, but are particularly important for an emerging industry such as the cannabis industry. The evolving legal and regulatory framework in the industry and the development of new technologies means that those businesses taking early steps to protect their intellectual property are likely to be at a competitive advantage as the industry matures and consolidates.
How do patents fit into the current state and federal cannabis regulations?
Currently, 29 U.S. states and the District of Columbia have legalized medical marijuana and eight states also permit recreational use. Under federal law, cannabis remains a Schedule I controlled substance under the Controlled Substances Act meaning that cannabis possession is illegal. However, the United States Patent and Trademark Office (USPTO), the federal agency responsible for granting patents, has traditionally taken a hands-off approach to barring particular kinds of inventions from patentability. The only kinds of inventions that are legally not patentable are inventions covering human life forms or whose sole purpose is use in atomic weapons. Otherwise, assuming that a patent application covering cannabis or cannabis-related products meets the legal standards for patentability, explained in more detail below, the USPTO presently grants patents to cannabis and cannabis-related inventions.
Source Lexology
Have you ever used a one-click ordering process online? Then you indirectly paid Amazon.
If you purchased anything from a website using a one-click purchase button, you indirectly paid Amazon for that ability, at least up until September 11, 2017 when Amazon’s patent to this technology expired. As a result, one-click purchasing might become the new norm. Continue reading “Have you ever used a one-click ordering process online? Then you indirectly paid Amazon.”
This Time-Saving Patent Paved the Way for the Modern Dishwasher
While hand washing the dishes has its upsides–it’s a meditative pastime that sometimes saves water–anyone who does it regularly can tell you it has its downsides too. For one thing, slippery plates sometimes get dropped and broken, ruining the symmetry of your four-serving set. For another, it can be time-consuming.
Continue reading “This Time-Saving Patent Paved the Way for the Modern Dishwasher”
Allergan Plc (NYSE:AGN) Shareholders Get Respite Against Ackman, Loses Patent To Sandoz Inc.
Allergan plc (NYSE:AGN) shareholders got a respite in case against William Ackman, his hedge fund, and Valeant Pharmaceuticals International Intl Inc (BMV:VRXN) recently when federal Judge David O. Carter rejected two major legal arguments from the side of Ackman. In another settlement, Allergan lost a patent battle with Sandoz Inc. over the Combigan eye drug.
Appeals court denies Regeneron bid to revive patent case against Merus
A federal appeals court on Tuesday refused to reconsider its ruling that a patent Regeneron Pharmaceuticals Inc asserted against rival Merus NV is unenforceable because it was obtained through deception. Continue reading “Appeals court denies Regeneron bid to revive patent case against Merus”